Immuno-oncology (IO) therapy is a new (and old) way to treat cancer by activating your immune system in the hope that it will attack your tumour.
One of the earliest treatments for cancer was a kind of immune therapy. Over a century ago, the American physician William Coley concocted a soup of bacterial toxins that was injected directly into tumours. The so-called “Coley’s Toxins” caused a great deal of inflammation and unfortunately sickness but did cure of a small number of people, and were used as treatment until well into the 1960s. Since then, a number of other immune therapies have been developed and trialled over the last century, but until recently side effects, cost and variable benefits have limited their usefulness.
Powered by our expanding knowledge of the cellular mechanisms of the immune system, newer types of immune therapies (also called immuno-oncology or IO therapies) have been proving successful in some types of cancer, including kidney cancer. Other types of immune therapies including vaccines are currently in trials for kidney cancer.
In this section, you will learn about which IO therapies effective for kidney cancer, how they work and what their side effects might be. To understand the new IO therapies it is important to know how our immune system works. If you want to learn more about our immune system, please read here first: How does the immune system work
Lymphocytes attacking cancer cell
Our immune system is able to recognise cancer cells as abnormal, and is sometimes able to mount an effective response against the cancer. In very rare cases of melanoma and kidney cancer, this can lead to a spontaneous remission, a miracle cure of the cancer. However, cancers are able to grow in the first place by making themselves invisible to the immune system, blocking anti-cancer signals that would otherwise cause the immune system to attack. The goal of immunotherapy is to activate, strengthen and support the body’s immune system against cancer.
There are several kinds of immune therapies available (and being tested) for many types of cancer:
1. Vaccines directed at cancer
Vaccines have been tested in many types of cancer, but older vaccines have mostly been ineffective in treating cancer to date. Trials are ongoing with new types of cancer vaccines. The most vigorous area of investigation are personalised therapies that use tissue from a patient’s tumour to create a customised vaccine for directing the immune system on targets expressed by their own cancer cells.
Note: Other hugely important immune-therapy vaccines are those that can prevent the viruses that cause specific cancers (such as hepatitis B and HPV – the human papilloma virus). These vaccines prevent hundreds of thousands of cancers around the world every year.
2. Cytokines (immune hormones)
The immune system communicates by hormones (e.g. interleukin and interferon). Large doses of these given as injections have been used to treat some kinds of cancer including kidney cancer.
3. Immune cells harvested and expanded out of the body
A person’s own immune cells (their lymphocytes) can be harvested from their body, and expanded outside the body and reintroduced. These include tumour infiltrating lymphocytes (TILs) and with further modification chimeric antigen receptor (CAR) T-cell therapy.
4. Checkpoint antibodies
So called monoclonal antibodies have been developed to disrupt the handshake between immune cells and their targets. This is a rapidly developing field for immune therapy of cancer.
Clinical trials are exploring how to personalise treatment based on the exact characteristics of your tumour. Some clinical trials request a biopsy or specimen of your tumour. Researchers are hoping to determine which types of cancers might be most receptive to IO therapies.
Each type of immune therapy uses different strategies to activate your immune system to fight your tumour.
1. Cancer vaccines
Cancer vaccines are given only after you have developed a tumour. Therefore these vaccines are therapeutic rather than preventive. They work by alerting the immune system to the cancer, and informing the immune system of targets to attack. Sometimes cancers can grow undetected by the immune system. A vaccine alerts the immune system to “find this abnormal cell”. There are several kidney cancer vaccines being tested in clinical trials. Although they all work by teaching the immune system how to recognise kidney cancer cells, they each do this in slightly different ways.
2. Cytokine therapy (immune hormones)
Immune hormones are made naturally in the body. These are messages that cells give each other, and they can cause immune cells to grow and become active. Massive doses of these can be given as an immune therapy, in the hope that it will activate enough of the immune system to attack the cancer. The cytokine interleukin-2 (IL-2) has been used to treat kidney cancer in some countries since the early 1990s. A lot of research has been carried out since then on how the immune system works and which cytokines might activate the immune system in a way that fights cancer more effectively than IL-2. Trials of the cytokines IL-10 and IL-21 as possible treatments for kidney cancer are underway. The future role of cytokines in the world of immuno-oncology for kidney cancer is not yet known.
3. Immune cell therapy
Immune cell therapy harvests immune cells from the blood or tumours of cancer patients, grows them in the lab, and then injects them back into patients. If the number of immune cells vs. tumour cells can be increased then it is hoped that this will lead to an attack on the cancer. These types of therapies are currently in clinical trials for some types of cancer, but are only available in a few major research institutions worldwide.
4. Checkpoint antibodies
Checkpoint antibodies are one of the newest forms of cancer immune therapy. While vaccines help the immune system identify the target, immune hormones activate immune cells and immune cell therapy expands the number of immune cells available, checkpoint antibodies lift the last line of defence that cancer cells use to defend themselves from the immune system. For further information on how checkpoint inhibitors work, please see the following questions.
At the moment there are four types of IO therapy being used and tested for kidney cancer:
1. Cancer vaccines (e.g., dendritic cell vaccines, others)
 Vaccines have been tried in kidney cancer. One vaccine seemed to be successful in preventing the cancer recurring in patients who had a large cancer removed from their kidney. This was a complex process however and this vaccine has not become a standard treatment.
Vaccines have been tried in kidney cancer. One vaccine seemed to be successful in preventing the cancer recurring in patients who had a large cancer removed from their kidney. This was a complex process however and this vaccine has not become a standard treatment.
Vaccines being tested in kidney cancer trials include:
- AGS-003: samples of your immune cells are collected and then genetically modified so that they recognise your tumour cells. Your modified immune cells are injected back into you. This is similar to CAR therapy, except that in this case the injected cells do not fight the tumour, but instead teach your adaptive immune system to fight it. This study did not show an improvement in outcome and is unlikely to become a standard treatment for kidney cancer.
- IMA901: this vaccine contains 10 common kidney cancer proteins. It works in a similar way to a normal vaccine, so it will not make you ill, but it is designed to teach your immune system how to recognise your tumour cells. IMA901 did not show a survival advantage to standard sunitinib treatment and will not be a standard treatment for kidney cancer.
- MGN1601: this vaccine contains someone else’s kidney cancer cells. When you are injected with the vaccine, your immune system will destroy these cells because they do not belong to you and will be recognised as a threat. While your immune system is fighting these foreign kidney cancer cells, it will also learn what a kidney cancer cell looks like and will therefore be able to destroy your tumour. This agent is still undergoing study.
2. Cytokine therapy (immune hormones for unspecified immune stimulation, e.g., IL2, IFN)
Before targeted therapies became available to patients with kidney cancer in 2006, some people were treated with immune hormones such as interleukin-2 (IL-2) and interferon-alpha (IFN). Some people who were given this older type of immuno-therapy responded very well to this treatment. Some people even had a durable complete response, meaning that the cancer went away completely and they have had a very long-term benefit from the treatment.
It is hard for doctors to use the word “cured” in this situation, but some people have lived a very long time after treatment. On the other side of the coin these immune hormones are extremely toxic; only young, physically fit people can take them, and even then people are so sick they are usually treated in intensive care units.
3. Immune cell therapy (e.g., T-cell infusion therapy or TILS)
These types of therapies are currently in clinical trials for some types of cancer, but are only available in a few major research institutions worldwide.
4. Checkpoint antibodies (e.g., CTLA-4, PD-1, PD-L1)
Checkpoint immune therapy is the most recent development in the immune therapy of kidney cancer. Many clinical trials are underway around the world that are either specifically for kidney cancer, or that include kidney cancer in the selection criteria, and several agents are now approved.
Immune cells are controlled by a committee of checkpoints which very finely balance the immune system. Too little regulation and the immune system goes overactive and attacks the body; too much and immune functions are impaired and infections and cancers can establish themselves.
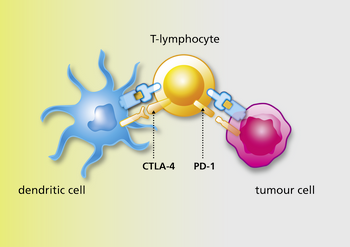 Sometimes cancer cells co-opt this system and use checkpoints inappropriately to stop your immune system recognising cancer cells. Checkpoint inhibitors are drugs (in this case antibodies) that in turn block these checkpoints so that the immune system is freed up to fight cancer cells.
Sometimes cancer cells co-opt this system and use checkpoints inappropriately to stop your immune system recognising cancer cells. Checkpoint inhibitors are drugs (in this case antibodies) that in turn block these checkpoints so that the immune system is freed up to fight cancer cells.
Understanding how these checkpoint antibodies work explains a lot about how they do (and don’t) work in cancer patients. For example, checkpoint immune therapy does not seem to help unless the immune system is already targeted, alerted, activated and attempting to infiltrate that cancer.
Researchers are still learning who will be and who won’t be helped, but it does seem that if there are no immune cells inside the tumour, then the checkpoint antibodies may not work. Understanding that checkpoint antibodies only lift the last line of the cancer’s defence against the immune system also explains the strategy that doctors and scientists are taking forward in clinical trials.
This work resulted in the US Food and Drug Administration (FDA) approval of nivolumab, a PD-1 blocking antibody, in 2015 for metastatic kidney cancer in the second-line setting, with subsequent approval in Europe and other parts of the world. Additionally the combination of nivolumab and ipilimumab, an antibody against CTLA-4 was US FDA approved in April 2018.
Depending upon where you live, checkpoint inhibitor drugs may be approved for use in kidney cancer. Others are available through clinical trials primarily also through compassionate use programs in some countries.
Types of Checkpoint IO Treatments
You may have heard the term “PD-1” and “PD-L1” and also “CTLA-4” as new types of medications being tested for kidney cancer. These are not drug names themselves, but indicate a type or “class” of checkpoint inhibitors that include medications such as nivolumab, pembrolizumab, ipilimumab, tremelimumab, durvalumab, avelumab and atezolizumab and others.
PD-1 and PD-L1 Inhibitors
These IO treatments include medication names such as nivolumab or pembrolizumab, and others in clinical trials such as durvalumab, avelumab and atezolizumab. A protein called PD-1 is present on the surface of immune system cells and its partner PD-L1 is present on the surface of other cells in the body.
PD-L1 on tumour cells allows them to stop the immune system from attacking them. If these two proteins bind, the immune cell thinks the other cell is healthy and moves on. This is effectively like a secret handshake so your immune system cells and healthy cells know that they are both on the same team. Tumour cells can trick the immune system into thinking that they are healthy cells by placing PD-L1 proteins on their surface: the cancer cell effectively learns the secret handshake.
PD-1 and PD-L1 inhibitors work by preventing PD-1 and PDL-1 from binding. This stops the cancer cell from being able to trick the immune system
CTLA-4 Inhibitors
These IO treatments include medication names such as ipilimumab and tremelimumab and others to be developed. Antibodies like ipilimumab and tremelimumab work by blocking the CTLA-4 checkpoint protein. CTLA-4 is another kind of checkpoint, but is not on tumour cells, rather it is on other immune cells. CTLA-4 stops the immune system from making too many tumour-fighting cells (the commando killer T-cells).
By blocking CTLA-4 with ipilimumab or tremelimumab, your immune system can hopefully make more of these cells to fight your tumour.
Combination therapies
The recent approval of combination nivolumab and ipilimumab for frontline treatment of metastatic kidney cancer has established combination IO therapy as a new standard of care for kidney cancer. While we do not have direct comparisons between this combination approach and single-agent IO treatment, the information that is being generated suggests the combination of two IO agents or of an IO agent with a targeted TKI type of drug may be the most effective treatment for patients with metastatic kidney cancer.
A number of clinical studies will produce results in the next few years, helping us answer this important question.
No. The word ‘cure’ is often used in the media but rarely in cancer centres by experts in the disease. It is too early to tell at the moment how effective these treatments will be in kidney cancer and which patients will see the most benefit.
But IO therapies are certainly one of the biggest breakthroughs in cancer treatment for a very long time.
It is not sensible to talk about a “cure” unless a patient’s cancer disappears completely and doesn’t come back for 5, 10, 15 years. The first checkpoint antibody was only shown effective in melanoma in 2010. However, the long-term survival of some of the earliest treated patients in these trials with melanoma is very encouraging. Several trials are ongoing with different forms of IO therapies.
Will immune therapy work for me?
We don’t know.
Everyone hopes that treatment will work for them, but at this stage it is not possible to predict who will, and who will not benefit from immune therapy. This is unfortunately the same situation as for standard treatments for kidney cancer such as blood-supply blocking drugs (e.g. sunitinib and pazopanib).
Many researchers are working to discover what will predict benefit from IO therapy, whether it is a feature of the cancer or of the patient himself or herself. This would allow us to predict who should not take IO therapy, but more importantly help us discover ways to extend the benefit of these therapies to more patients.
There are many questions that remain unknown about IO therapy. Who should take the therapy? Who shouldn’t take it? Does a patient need a single checkpoint antibody or would a combination be better? If the treatment works, how long would you need to take the treatment? Is 12 weeks enough or does one need a year or two years of treatment? How long do you take the treatment before assessing that it has not helped?
Assessing whether IO therapy has helped an individual patient is difficult. Because the treatment does not directly attack the cancer, and one must wait for the immune system to act, many people who benefit have a delayed response. They may need to take several doses of the treatment before seeing an improvement. Some people even see their cancers worsen substantially before later improving. Up to 20% of people taking ipilimumab for melanoma, and up to 5% of people taking PD-1 antibodies like pembrolizumab or nivolumab in many cancers experience this pseudoprogression.
If IO therapy does give benefit, sometimes this is substantial, with all cancer spots resolving in the body, leaving no evidence on scans that the cancer remains. This is called a complete response, and is not common. A partial response is when cancer spots are still visible on the scan but all of them are smaller. This is often also a very good situation as spots left on a scan might be just scar tissue. However there is a third outcome of IO therapy that is also associated with patient benefit and improved survival, so called stable disease. Unsurprisingly stable disease means just that; there are no new spots of cancer, and though the cancer spots are not much smaller, none of them are bigger. The reason why this is beneficial in IO therapy is that this stability is often long-lived; and so too is the patient, who can live for long periods of time, still with the cancer clearly visible on the scan.
Your doctor will order scans before you start treatment but will often wait 2 or even 3 months before doing another set of scans as there is no point doing scans early, as the benefits of IO therapy are often delayed. Even if the cancer seems to be getting worse your doctor may recommend continuing for another 1-2 doses and repeating scans, as again pseudoprogression can occur.
The development and availability of checkpoint antibodies for different cancers has been one of the most rapid stories in medical history. It has been a whirlwind of activity and new findings are published every month.
 First and foremost, patients should seek advice from an expert in kidney cancer to determine whether immuno-oncology treatment is appropriate for them.
First and foremost, patients should seek advice from an expert in kidney cancer to determine whether immuno-oncology treatment is appropriate for them.
Kidney cancer experts can best advise you on various ways to access immuno-oncology therapies for kidney cancer. Depending upon your country, these methods may include:
Obtain IO agents through your health care system
Nivolumab and the combination of nivolumab and ipilimumab may be available through your health care system in your country. Check with your physician team to determine what is available to you at this point in time.
Join a clinical trial
Different types of IO therapy for kidney cancer are being actively researched in clinical trials. This website maintains a list of current IO clinical trials for people with kidney cancer. Trials included are those operating in more than one hospital or recruiting a significant number of patients. Smaller trials might also be available at your hospital. Some are in the final phases (Phase 3). If these trials are successful, the first medications may be approved for use.
However, depending upon your country, the clinical trial and approval processes can take some time so it may be several years before any type of IO therapy becomes widely available. In the meantime, new trials are expected to answer important questions specific to kidney cancer.
Seek Compassionate Access Programs
Some checkpoint antibodies may be available on compassionate grounds. Some companies have established compassionate access programs, or expanded access programs where doctors can apply for early access to drugs. This is complex, varies by country, is subject to change at short notice and requires a lot of paperwork. The patient organisation in your country may know which compassionate access programs are available. For information on patient organisations near you, please check “our global network“.
Obtain through Private Insurance or Personal Funds
The cost of these new drugs is very high. Estimated costs could be as much as $150,000 U.S. for one year. Insurance companies might or might not cover the cost of these medications. Depending upon your country’s regulations, you might or might not be able to pay for the drug personally and have it infused. Be sure to speak to your physician before paying out of pocket for the medication to ensure that there are no clinical trials or compassionate access programs that you could benefit from.
As a kidney cancer patient, you should be aware that the side effects of immuno-oncology treatments might be very different from side effects that you have experienced before on any prior therapies. For this reason, it’s important to understand a few basic facts about experiencing and reporting immuno-oncology side effects.
